Most Read
Abstract
Undifferentiated connective tissue disease has been used to describe disorders characterized by the presence of systemic autoimmune disease signs and symptoms that do not meet the diagnostic criteria for a recognized illness. Strongyloidiasis is a parasitic infection caused by Strongyloides stercoralis that lasts for years. We present a case with the first discovered undifferentiated connective tissue disease presented by pulmonary strongyloidiasis apart from hyperinfection syndrome.
INTRODUCTION
Strongyloidiasis is a parasitic infection caused by Strongyloides stercoralis that lasts for years. It is found in tropical and subtropical regions (1). Skin contact with contaminated soil, as well as unsanitary settings, are risk factors for infection. In warm and damp soil, rhabditiform larvae can grow into filariform larvae that can enter human skin and travel through circulation to the lungs. Patients are often asymptomatic and capable of controlling larval growth. The parasite, on the other hand, is capable of autoinfection, which allows it to proliferate in the same host in the absence of further external infestation (2).
Autoinfection can result in a vast and widespread life-threatening parasite infestation known as hyperinfection syndrome (HS). Several organs might be involved in HS, but respiratory and gastrointestinal systems are affected in most cases. Strongyloidiasis is frequently difficult to diagnose in non-endemic areas (3). People with advanced age, malnutrition, immunosuppressive medications such as steroids, diabetes mellitus, and human T-cell lymphotropic virus type 1 (HTLV-1) can develop HS (4).
Since 1980, the term ‘undifferentiated connective tissue disease’ (UCTD) has been used to describe disorders characterized by the presence of systemic autoimmune disease signs and symptoms that do not meet the diagnostic criteria for a recognized illness. UCTD, like other connective tissue disorders, manifests itself in the middle years of life (5). UCTD may persist indefinitely for years. This is described in around 70% of the known cases in the literature. It may exhibit a spontaneous or drug-induced clinical regression or a gradual evolution to a recognized connective tissue disease, primarily represented by systemic lupus erythematosus (SLE) (5,6).
We present a case with the first discovered UCTD, which was presented by pulmonary strongyloidiasis without HS.
CASE
A 27-year-old male engineer was brought to the emergency department with complaints of fever, shortness of breath, and coughing up blood for 14 days. The patient was not a known diabetic or hypertensive and was not on any medication in the recent past or at the time of admission.
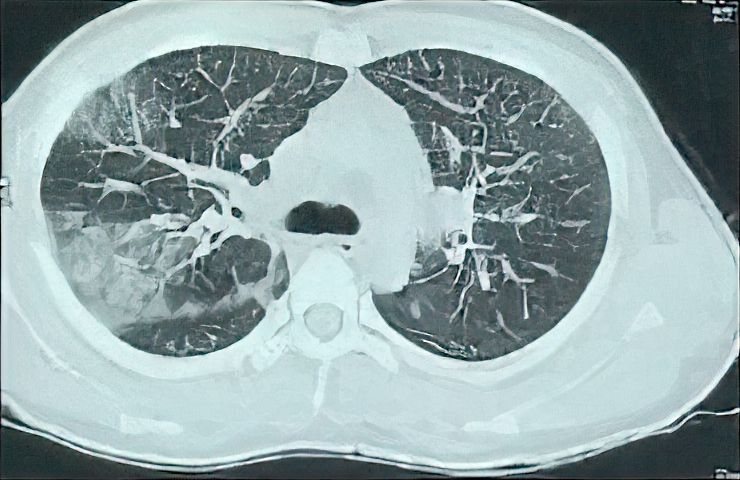
Figure 1. The Initial CT of the Patient Shows Interlobular Septal Thickening, Right Upper Lobe Consolidations and Ground Glass Opacity.
On examination, blood pressure was 120/80 mmHg, pulse rate 110/min, peripheral oxygen saturation (SpO2) 80% without oxygen, and respiratory rate 32/min. No abnormalities of the abdomen or central nervous system were observed. There were scattered crepitations in both infrascapular areas and the right inframammary area. High-resolution chest CT (HRCT) showed bilateral ground glass opacities, which were more obvious in the posterior segment of the right upper lobe and middle lobe in addition to the bilateral interlobular septal thickening (Figure 1).
The biochemical parameters (random blood sugar: 135 mmol/L, albumin: 3.4 mg/dL, serum bilirubin: 0.8 mg/dL, ALT: 15 U/L, creatinine: 1.2 mg/dL, K: 4.3 mmol/L) were in normal limits. Complete blood count revealed elevated white blood cells (WBC) (37 000/µL), platelets (127 000/µL), and hemoglobin (10.4 g/dL). INR (1.1), D-dimer (0.2 g/L) and LDH (294 IU/L) were within normal limits. CRP (48 mg/L) and ESR (35 mm/hr) were slightly elevated. PCR for COVID-19 was negative for twice.
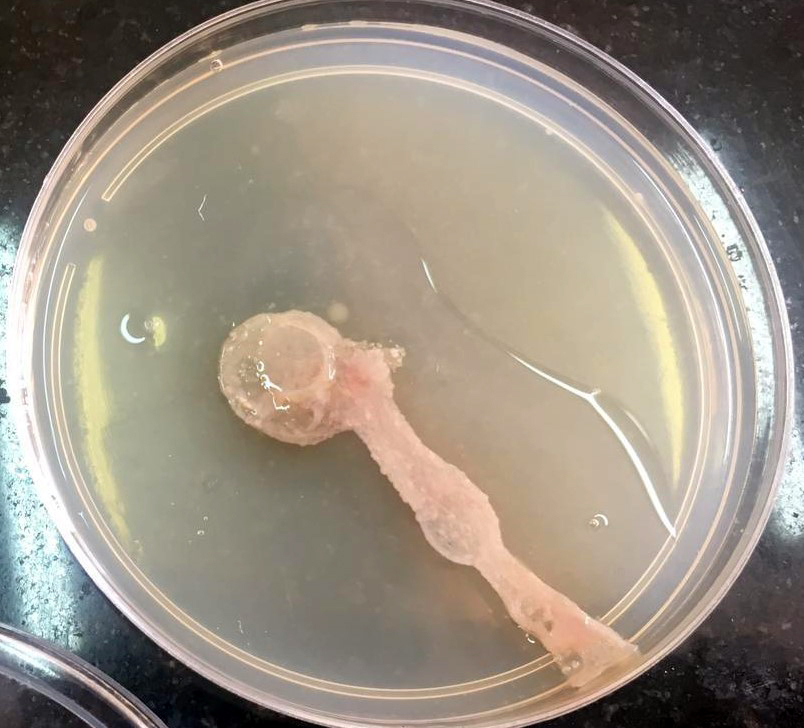
His echocardiography was within normal limits apart from pulmonary systolic pressure of 45 mmHg. Screening tests for HBsAg and HCV antibodies were negative. Based on history and HRCT findings, the patient was provisionally diagnosed as a case of mild COVID-19 infection. He was not given corticosteroid treatment during COVID-19 infection. RT-PCR for COVID-19 was negative twice at 48 hours intervals. On day 4 of admission, screening tests for autoimmune disorders (ANA, anti-ds DNA, ANCA c, ANCA p, anti-cardiolipin antibodies, anti-basement membrane antibodies, direct and indirect coombs test) were negative apart from low C3 (30 mg/dL) and urine analysis revealed proteinuria (600 mg). However, during admission, WBC increased to 65 000 per microliter, then 130 000 per microliter, with peripheral eosinophilia of 40%. An increase in WBC was associated with a drop in hemoglobin and platelets to 7.5 g/dL and 40 000 per microliter, respectively. Peripheral blood smear, bone marrow aspiration, and biopsy revealed toxic granules, and hematological malignancies were excluded. The rheumatology and immunology department diagnosed the patient as having undifferentiated connective tissue disease based on clinical and laboratory data. Corticosteroid (methylprednisolone 500 mg IV daily for four consecutive days) was started for undifferentiated connective tissue disease. The patient had diarrhea and a painful knee joint with limitation of activity during hospital admission. CT angiography of the abdomen showed patent abdominal vessels, and knee effusion was found in the knee ultrasound. Tropical disease consultation advised stool and sputum analysis to exclude parasitic infestations, especially with the presence of eosinophilia. Sputum and stool culture (single sample) revealed migratory tracts on agar plates, suggesting Strongyloides stercoralis infection (Figure 2). After extensively checking the possible immunocompromised condition of the patient, human HTLV-1 infection, HIV antibody, and immunoglobulin levels were negative. Albendazole (400 mg/day in divided doses for three days) was started, followed by improvement of clinical parameters of the patient (respiratory rate 20/minute, SpO2 98% without oxygen) and laboratory parameters (WBCs 10 000 per microliter, eosinophil 5%, CRP negative, ESR 5 mm/hr.). Despite these improvements in clinical and laboratory data, HRCT did not improve, and the patient developed neurological symptoms with an MRI diagnosis of autoimmune encephalitis.
DISCUSSION
The presence of larvae in stool specimens or respiratory secretions is always used to diagnose strongyloidiasis (7). S. stercoralis was not found in our patient’s stool or sputum. Stool examination sensitivity is limited, particularly in non-endemic areas (8). The migratory tract on the surface of the agar plate gave the first clue for diagnosis in this case. Cheong et al. (9) discovered one tract generated by larva migration pushing bacteria away from the initial culture lines on the surface of an agar plate, and S. stercoralis was further detected from the sputum and stool specimen. In Bae et al. (8), stool examination also failed to reveal S. stercoralis.
Ground-glass opacity and interlobular septal thickening were observed in our case, which are common findings on chest CT in pulmonary strongyloidiasis. This CT observation shows that alveolar hemorrhage might be employed as a possible marker for the shift from asymptomatic to symptomatic condition (4). The number of larvae in the host of HS patients constantly increases. As a result, numerous larvae penetrate through the alveolar membranes, causing massive tissue damage in some cases. This is thought to be the cause of bacterial pneumonia and alveolar hemorrhage (10).
Microfilariae move from pulmonary capillaries into the alveoli during the migratory phase. This migration frequently results in peripheral eosinophilia. Eosinophilia of more than 5% has been documented in 65-90% of individuals, with a mean eosinophil percentage ranging between 13% and 18% (10). Numerous studies have assessed the value of eosinophilia in the diagnosis of S. stercoralis. Loutfy et al. (11) showed that in patients with confirmed S. stercoralis infection, 82.6% of the patients had eosinophilia, suggesting eosinophilia as a potentially useful marker for the diagnosis of the infection. In our case, peripheral eosinophilia of 40% was found. However, the absence of eosinophilia is a poor prognostic indicator (12).
Immunocompromised patients are at risk of HS, in which the organism quickly proliferates and disseminates throughout the host. Yung et al. (13) described an atypical occurrence of HS in a patient receiving continuous corticosteroid therapy for systemic lupus erythematosus (SLE). Singh et al. (14) also described a case of hyperinfection with S. stercoralis presenting as acute abdomen in a known case of mixed connective tissue disorder on corticosteroid therapy. In accordance with our case, Singh et al. (14) patient also showed negative stool microscopy, and a definitive diagnosis was done only on histopathology.
S. stercoralis hyperinfection was detected in a patient with rheumatoid arthritis who received immunosuppressive agents, including methotrexate and steroids. Stool and sputum parasitological examinations were also all positive for S. stercoralis larvae (15). Prior to starting immunosuppressive medication, it is critical to identify individuals who are at risk of infectious problems. The most well-known screenings are for latent tuberculosis infection (LTBI) and hepatitis B, whereas hepatitis C, human immunodeficiency virus (HIV), and Strongyloides infection should be examined on an individual patient basis (16).
Strongyloidiasis diagnosis could be challenging due to non-specific clinical features. Pulmonary strongyloidiasis should be included in the differential diagnosis of patients with undifferentiated connective tissue disease with ground-glass opacity and interlobular septal thickening in chest HRCT.
Informed Consent
Written informed consents for publication of their clinical details were obtained from the patients.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – H.A.; Design – H.A., K.R.; Supervision – A.H., A.E., S.A.; Materials – R.E., A.H., S.T.; Data Collection and/or Processing – K.R., H.A.; Analysis and/or Interpretation – H.A., H.E., S.T.; Literature Review – H.E., R.E., S.A., K.R.; Writer – H.A., A.E., S.A., R.E.; Critical Review – H.E., A.E., S.T., A.H.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
The authors declared that this study has received no financial support.
Acknowledgments
The authors thanks to the Chest Medicine Department, the Tropical Medicine Department, the Medical Parasitology Department, and the Rheumatology and Immunology Unit & Internal Medicine Department of Mansoura University.
References
- Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. [CrossRef]
- Lam CS, Tong MK, Chan KM, Siu YP. Disseminated strongyloidiasis: a retrospective study of clinical course and outcome. Eur J Clin Microbiol Infect Dis. 2006;25(1):14-8. [CrossRef]
- Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides Am J Trop Med Hyg. 1995;53(3):248-50. [CrossRef]
- Nabeya D, Haranaga S, Parrott GL, et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect Dis. 2017;17(1):320. [CrossRef]
- Conti V, Esposito A, Cagliuso M, et al. Undifferentiated connective tissue disease – an unsolved problem: revision of literature and case studies. Int J Immunopathol Pharmacol. 2010;23(1):271-8. [CrossRef]
- Mosca M, Neri R, Bencivelli W, Tavoni A, Bombardieri S. Undifferentiated connective tissue disease: analysis of 83 patients with a minimum follow up of 5 years. J Rheumatol. 2002;29(11):2345-9.
- Marchi Blatt J, Cantos GA. Evaluation of techniques for the diagnosis of Strongyloides stercoralis in human immunodeficiency virus (HIV) positive and HIV negative individuals in the city of Itajaí, Brazil. Braz J Infect Dis. 2003;7(6):402-8. [CrossRef]
- Bae K, Jeon KN, Ha JY, Lee JS, Na BK. Pulmonary strongyloidiasis presenting micronodules on chest computed tomography. J Thorac Dis. 2018;10(8):612-5. [CrossRef]
- Cheong CU, Gau SJ, Lai CC. Strongyloides stercoralis in sputum. QJM. 2014;107(3):235-6. [CrossRef]
- Mokhlesi B, Shulzhenko O, Garimella PS, Kuma L, Monti C. Pulmonary strongyloidiasis: The varied clinical presentations. Clin Pulm Med. 2004;11(1):6-13. [CrossRef]
- Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66(6):749-52. [CrossRef]
- Upadhyay D, Corbridge T, Jain M, Shah R. Pulmonary hyperinfection syndrome with Strongyloides stercoralis. Am J Med. 2001;111(2):167-9. [CrossRef]
- Yung EE, Lee CM, Boys J, Grabo DJ, Buxbaum JL, Chandrasoma PT. Strongyloidiasis hyperinfection in a patient with a history of systemic lupus erythematosus. Am J Trop Med Hyg. 2014;91(4):806-9. [CrossRef]
- Singh K, Ganorkar S, Bhalekar S, Rao R. Hyperinfection with Strongyloides stercoralispresenting as acute abdomen in a patient on corticosteroid therapy: A case report. Indian J Pathol Microbiol. 2021;64(4):831-3.
- Altintop L, Cakar B, Hokelek M, Bektas A, Yildiz L, Karaoglanoglu M. Strongyloides stercoralis hyperinfection in a patient with rheumatoid arthritis and bronchial asthma: a case report. Ann Clin Microbiol Antimicrob. 2010;9:27. [CrossRef]
- Jee AS, Sheehy R, Hopkins P, et al. Diagnosis and management of connective tissue disease-associated interstitial lung disease in Australia and New Zealand: A position statement from the Thoracic Society of Australia and New Zealand. Respirology. 2021;26(1):23-51. [CrossRef]